This video is a gift from Dr Satoshi Inouye, at Chisso Corprotaion Research in Yokohama, Japan. Movie is wiith permission: Suzuki T et al, PLoS ONE 6, e25243, 2011
AWESOME real time single cell imaging (PubMed)
E-Coelenterazine an optimized Renilla Luciferin
Introducing e-Coelenterazine, an optimized Luciferin for Renilla Luciferases
E-Coelenterazine or Coelenterazine E was first described in 1989 by Nobel prize laureate Dr. Osamu Shimomura. The big advantage of this Coelenterazine analogue is an initial increase in light intensity by 750% and an overall increase of light emission of 137%
JUST ADD WATER Coelenterazine YES!
NanoLight™ has a new proprietary injectable Coelenterazine!
Used with Gaussia luciferase and Renilla luciferases,
Works just like native Coelenterazine but MORE SIGNAL!
Comes sterile and in perfect sizes for in vivo mouse imaging:
Check this comparison out:
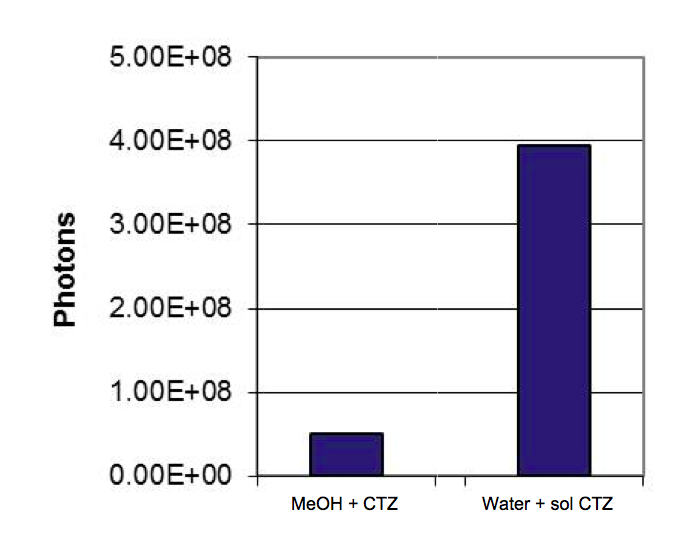
100 ug I.V. injection into mouse comparing water soluble to MeOH dissolved CTZ.
Gaussia luciferase is used as a reporter in conjunction with CRISPR
Prolume Purple in BRET Applications
Prolume Purple (Cat. #369) enabled these researchers to follow protein movement within a single cell in real-time for up to 20 min using a sophisticated BRET system.
July 2016 Nature Communications Article: Monitoring G protein-coupled receptor and beta-arrestin trafficking in live cells using enhanced bystander BRET Yoon Namkung, Christian Le Gouill, Viktoria Lukashova, Hiroyuki Kobayashi, Mireille Hougue, Etienne Khoury, Mideum Song, Micharl Bouvier, Stephane A. Laporte
Abstract
Endocytosis and intracellular trafficking of receptors are pivotal to maintain physiological functions and drug action; however, robust quantitative approaches are lacking to study such processes in live cells. Here we present new bioluminescence resonance energy transfer (BRET) sensors to quantitatively monitor G protein-coupled receptors (GPCRs) and β-arrestin trafficking. These sensors are based on bystander BRET and use the naturally interacting chromophores luciferase (RLuc) and green fluorescent protein (rGFP) from Renilla. The versatility and robustness of this approach are exemplified by anchoring rGFP at the plasma membrane or in endosomes to generate high dynamic spectrometric BRET signals on ligand-promoted recruitment or sequestration of RLuc-tagged proteins to, or from, specific cell compartments, as well as sensitive subcellular BRET imaging for protein translocation visualization. These sensors are scalable to high-throughput formats and allow quantitative pharmacological studies of GPCR trafficking in real time, in live cells, revealing ligand-dependent biased trafficking of receptor/β-arrestin complexes.


